RESEARCH
OVERVIEW
The Smogorzewska laboratory studies how DNA is damaged and repaired during DNA replication. Most of our work to date explored the repair of DNA interstrand crosslinks (ICLs) that covalently link two strands of DNA and preclude proper replication and transcription. We study rare genetic diseases including Fanconi anemia and karyomegalic interstitial nephritis, which are characterized by abnormal ICL repair. Our ongoing studies aim to identify new genes, determine their role in DNA ICL repair, and illuminate the molecular pathogenesis of these diseases, including development of bone marrow failure, leukemia, squamous cell carcinoma, and kidney failure. We also develop mouse models to investigate the in vivo consequences of deficiencies in DNA repair.
Most recently, we initiated studies to understand how the replication machinery responds to what is commonly called “replication stress,” any situation that impedes normal movement of the replication fork. The goal is to understand events that unfold at the fork in order to produce daughter cells without inducing genome instability.
IDENTIFICATION OF NEW GENES NECESSARY FOR INTERSTRAND CROSSLINK (ICL) REPAIR
In the last six years, we have identified FANCP/SLX4, FANCR/RAD51, and FANCT/UBE2T. We identified FAN1 in an shRNA screen for proteins necessary for ICL repair. The Hildebrandt lab found FAN1 mutations in patients with karyomegalic interstitial nephritis and we showed that FAN1 deficiency was responsible for the cellular defects of the patients cells. Our lab continues to identify new genes involved in DNA repair.
IN VIVO CONSEQUENCES OF INAPPROPRIATE ICL REPAIR
We have made a mouse model of FAN1 deficiency and are studying the phenotypes of this mouse to gain better understanding of human disease.
DNA transactions at the ICL and the FANC proteins. The proteins encoded by the nineteen genes identified to be mutated in FA patients (colored in the schematic) are implicated in a common pathway important for the repair of DNA interstrand crosslink (ICL) lesions that covalently link two strands of DNA. Associated proteins that have not been shown to be mutated in FA patients are shown in grey. Upon replication fork stalling at the ICL, FANCI and FANCD2 are ubiquitinated by the FANCL in the core complex and localize to the ICLs where they directly or indirectly recruit effectors including SLX4, FAN1, and translesion polymerases. The repair is completed by homologous recombination. FANCS/BRCA1 has been also implicated in unloading of the replicative CMG helicase at stalled replication forks and RAD51 functions to protect the DNA at the crosslink from inappropriate degradation by DNA2/WRN complex. Letters stand for full gene names A=FANCA, B=FANCB, R=FANCR, and so on. Figure and text are adapted from Wang and Smogorzewska, SnapShot: Fanconi anemia and associated proteins. Cell 2015 Jan 15. This model is based on work from many labs working on ICL repair.
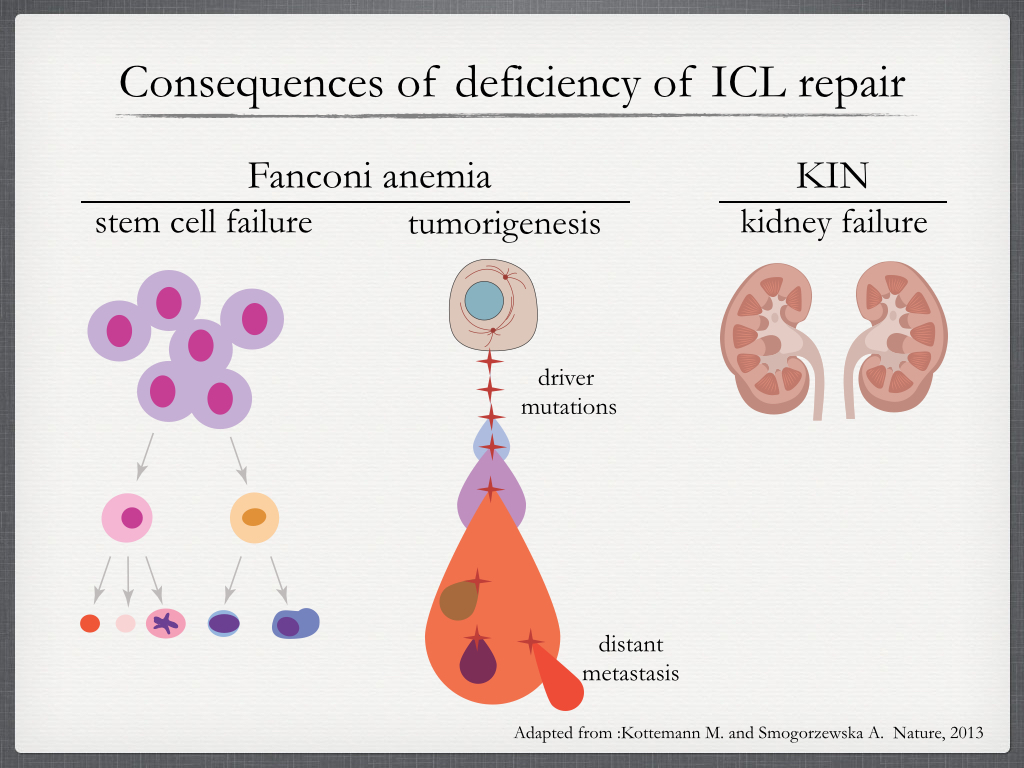
Consequences of poor ICL repair. Unrepaired or misrepaired ICLs lead to stem cell failure, tumorigenesis and kidney failure. Fanconi anemia (FA) patients may display diverse congenital abnormalities, including growth restriction, skeletal, and other organ malformations. Most FA patients develop bone marrow failure that may affect any of the blood lineages. FA patients are also cancer prone. Kidney failure is characteristic in karyomegalic interstitial nephritis (KIN) caused by mutations in FAN1, which codes for a nuclease that participates in ICL repair
MECHANISM OF ICL REPAIR
Patient cell lines are invaluable to identify mechanism of ICL repair. Especially useful are naturally occurring separation of function mutations that direct us to new functions of proteins.
UNDERSTANDING TUMORIGENESIS IN FANCONI ANEMIA
Fanconi anemia is a tumor predisposition syndrome. Patients have an increased risk (700 fold) to develop squamous cell carcinoma. The most prevalent cancer sites involve head and neck, anogenital area, esophagus, and lung. We are taking a genomic approach to understand the pathogenesis of these cancers with the hope of developing biomarkers and future treatments.
Examples of patient samples with the top left picture showing normal mucosa, the middle top picture showing transition of normal to cancerous area, and the other pictures showing tumors.